Except where stated otherwise, the electrolyte used was sodium chloride solution
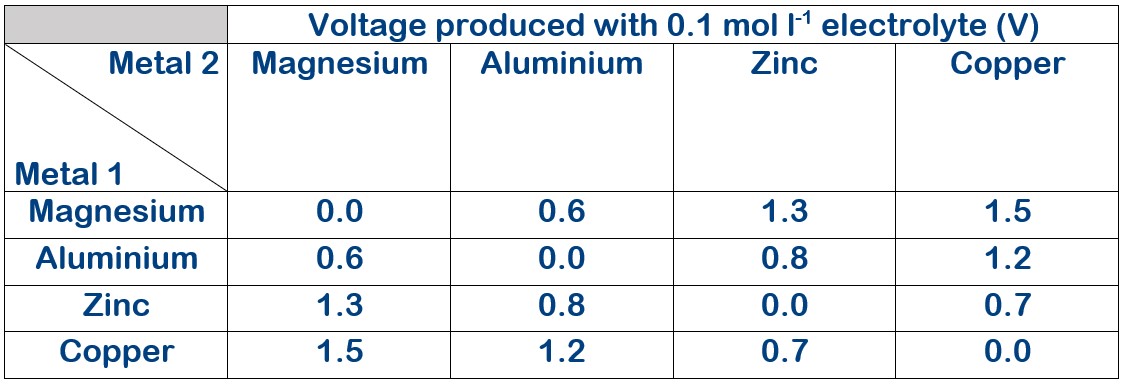
Electrolyte concentration 0.1 mol l-1
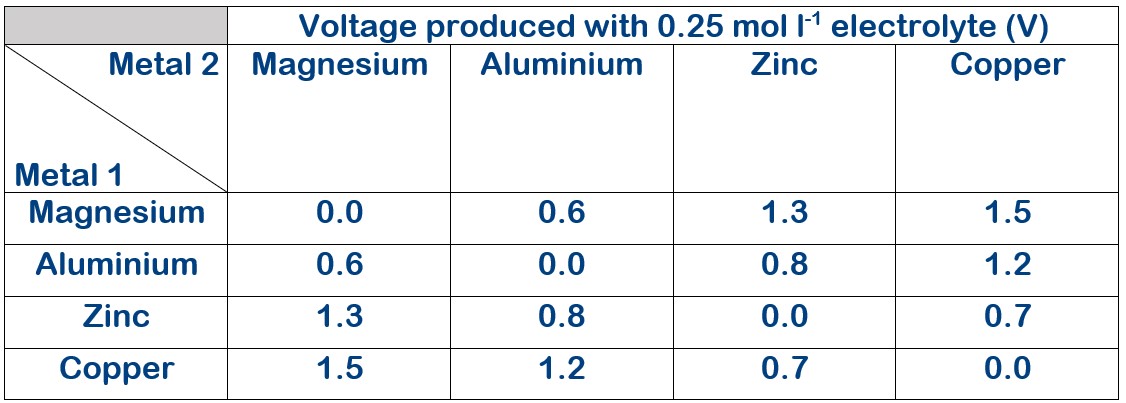
Electrolyte concentration 0.25 mol l-1
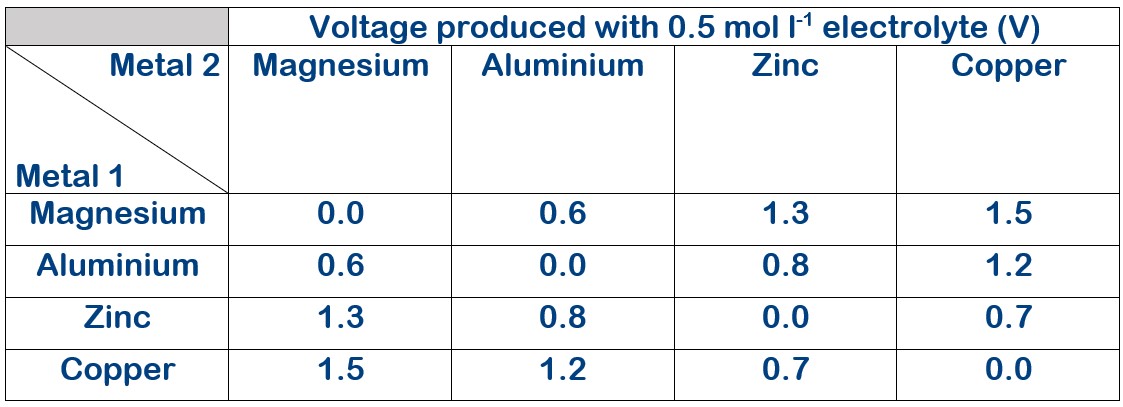
Electrolyte concentration 0.5 mol l-1
Electrolyte concentration 1.0 mol l-1
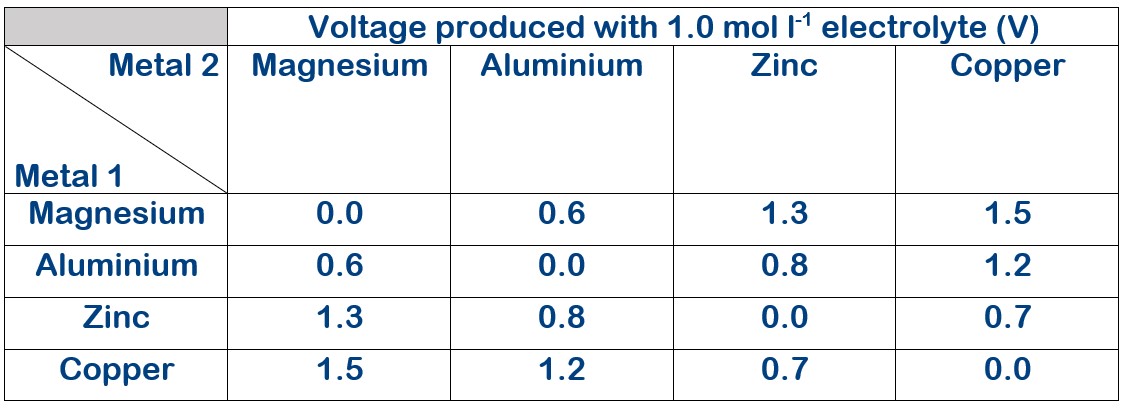
The voltage produced with varying electrolyte concentration.
and
All done using (Mg/Cu electrodes)
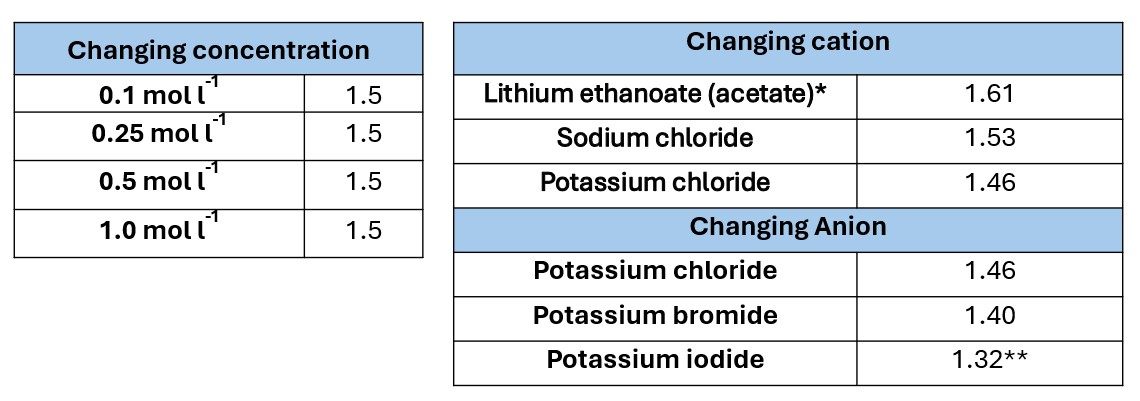
* No LiCl was available at the time. The reading is likely to be a little lower than would have been with the chloride. A comparison of Sodium chloride with sodium ethanoate showed voltages of 1.53 and 1.46 respectively
** The potassium iodide was slightly yellow so some of it had become iodine, lowering the concentration of iodide ions slightly.
Although the mechanism seems obscure, we suspect it has something to do with ion radius and mobility.
